Abstract
Assessment of minimal residual disease (MRD) in patients with complete remission CR) defined as <5% bone marrow blast count at allogeneic stem cell transplantation (HSCT) provides powerful independent prognostic information in acute myeloid leukemia and defines risk groups for transplant outcomes. Previous studies showed that transplant outcomes for AML patients with morphologically detectable disease (active disease) are comparable to those for CR patients with MRD (CR-MRDpos).
We aimed to compare the outcomes of 458 AML patients who underwent HSCT after 2012 at our institution based on the status of morphologically detectable disease and MRD by 8 color flow cytometry at HSCT in addition to cytogenetic and molecular risk classification by European LeukemiaNet. The primary outcomes were disease progression and progression free survival (PFS) and analyses were stratified by ELN risk classification as favorable and intermediate (n=295) and adverse (n=163).
Patients were categorized as active disease (n=81) vs. CR-MRDneg (n=191) and CR-MRDpos (n=52) vs. primary induction failure with marrow aplasia (PIF)-MRD (n=25) and PIF-MRDpos (n=74) vs. relapsed refractory with marrow aplasia (RR)-MRDneg (n=19) and RR-MRDpos (n=16). Median age of cohort was 56 (range, 3-80). Conditioning intensity was either myeloablative (n=260) or reduced intensity (n=198).
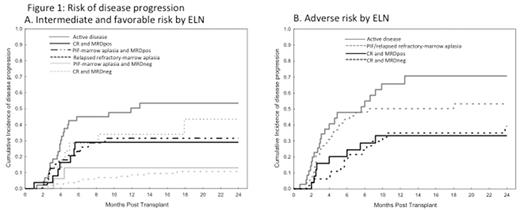
The median follow-up of survivors was 18 months. For disease progression in favorable/intermediate risk by ELN, the only prognostic factors were the morphological disease status and MRD as shown in Figure 1. Active disease patients had the highest incidence of progression at 2-year with 53% which was similar to 43% observed in RR patients (HR=0.6, p=0.3). In comparison to active disease, CR-MRDpos and PIF-MRDpos patients had lower incidence of progression with 29% and 32% at 2-yr (HR=0.5, p=0.08 and HR=0.5, p=0.06). Among adverse risk patients, CR patients had significantly lower risk of disease progression independent of their MRD status when compared with active disease patients (HR=0.4, p=0.002 for CR-MRDneg and HR=0.4, p=0.02 for CRMRDpos).
For PFS at 2 year, not only morphological disease status and MRD but also older age and hematopoietic comorbidity index (HCT-CI) were prognostic. In patients with favorable/intermediate ELN risk, Classification and Regression Tree multivariate analysis revealed 3 potential PFS groups: high PFS group included CR-MRDneg patients aged <60 who had the best 2-yr PFS estimated at 81%, and CR-MRDneg but aged ≥60 who had an estimated 2-yr PFS of 61%. In contrast, low PFS group included not CR-MRDneg patients with HCT-CI ≥4 in combination with either RR-marrow aplasia (2-yr PFS 0%) or age >60 (2-yr PFS 10%). In the absence of RR-marrow aplasia and older age, not CR-MRDneg patients with HCT-CI ≥4 had an intermediate 2-yr PFS (30%) which was comparable to the PFS (43%) in not CR-MRDneg patient with HCT-CI <4, irrespective of age.
For adverse ELN risk group, PFS was the best in patients with CR (independent of the MRD status) and HCT-CI <4 with 2 year PFS of 59%. All other adverse risk patients had similarly low PFS with estimates of 17%-23%.
In conclusion, we observed a lower progression incidence in CRMRDpos patients compared with active disease in both intermediate and adverse risk ELN groups after HSCT. However, PFS is a more complex endpoint with not only morphological disease status and MRD but also ELN risk classification, older age and HCT-CI determining the outcome expectations. These factors need to be taken into account when planning treatment for HSCT and thereafter.
Oran: Celgene: Research Funding; AROG: Research Funding; Astex: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal